1. Project synopsis.
Echocardiographic Evaluation of Hypertensive Acute Pulmonary Edema
This study has been completed.
First Received: January 26, 2009 Last Updated: June 29, 2009 History of Changes
Sponsor: | Carol Davila University of Medicine and Pharmacy |
Collaborator: | Ministry of Education and Research, Romania |
Information provided by: | Carol Davila University of Medicine and Pharmacy |
ClinicalTrials.gov Identifier: | NCT00829855 |
Acute cardiogenic pulmonary edema (ACPE), one of the most severe forms of acute heart failure, represents 5% of hospital admissions. One of the most frequent phenomena encountered during ACPE is hypertensive crisis (hypertensive ACPE) but the mechanisms and causes of hypertensive ACPE are insufficiently understood. Few studies have evaluated the cardiac function during hypertensive ACPE, and these studies used only conventional echocardiography methods. New methods of evaluation of cardiac function in hypertensive ACPE (such as Tissue Doppler imaging) have not been used.
The objectives of this study are to evaluate presence and role of the following potential mechanisms of hypertensive ACPE: 1. acute myocardial dysfunction (systolic and diastolic); 2. silent transient myocardial ischemia; 3. acute mechanical left ventricular dyssynchrony; 4. dynamic mitral regurgitation; 5. inter-ventricular interaction. Conventional and Tissue Doppler echocardiography will be used to assess cardiac function.
Study Type: | Observational |
Study Design: | Observational Model: Case-Crossover |
Official Title: | Echocardiographic Evaluation of Hypertensive Acute Pulmonary Edema |
Primary Outcome Measures:
.acute myocardial dysfunction (systolic and diastolic) and/or dyssynchrony [ Time Frame: acute event and 48 to 96 h after the event ] [ Designated as safety issue: No ]
Secondary Outcome Measures:
.surrogate markers of silent transient myocardial ischemia [ Time Frame: acute event and 48 to 96 h after the event ] [ Designated as safety issue: No ]
.dynamic mitral regurgitation [ Time Frame: acute event and 48 to 96 h after the event ] [ Designated as safety issue: No ]
.inter-ventricular interaction [ Time Frame: acute event and 48 to 96h after the event ] [ Designated as safety issue: No ]
Biospecimen
Retention: None Retained
Enrollment: | 51 |
Study Start Date: | May 2008 |
Study Completion Date: | May 2009 |
Primary Completion Date: | May 2009 (Final data collection date for primary outcome measure) |
1 Patients with hypertensive (systolic blood pressure ≥160 mmHg) acute pulmonary edema, evaluated within 120 minutes after admittance. |
2 The same patients from group 1 followed-up at 48 to 96 hours. |
Ages Eligible for Study: | 18 Years and older |
Genders Eligible for Study: | Both |
Accepts Healthy Volunteers: | No |
Sampling Method: | Probability Sample |
Study Population
Patients admitted in the Cardiac Care Unit of an University and Emergency Hospital, with a diagnosis of acute cardiogenic pulmonary edema associated with hypertension (systolic blood pressure ≥160 mmHg)
Criteria
Inclusion Criteria:
.acute onset of dyspnea within the preceding 8 hours
.respiratory distress and pulmonary rales at any level
.pulmonary congestion confirmed by chest radiography
.systolic blood pressure > 160 mmHg before treatment
.sinus rhythm
.signed informed consent
Exclusion criteria:
.acute myocardial infarction confirmed by myocardial necrosis markers (either CKMB or troponin I). Angina pectoris alone will not be excluded
.significant left sided valvular disease (more than moderate). Mitral regurgitation of any severity will not be excluded, due to the hypothesis of the role of dynamic mitral regurgitation in the pathogenesis of acute hypertensive pulmonary edema
.congenital heart disease
.cardiac tamponade
.rhythm and conduction disturbances that may have precipitated pulmonary edema, like sustained ventricular tachycardia and complete heart block
Please refer to
this study by its ClinicalTrials.gov identifier:
NCT00829855
Locations
Romania |
Department of Cardiology, University and Emergency Hospital of Bucharest |
Bucharest, Romania, 050098 |
Sponsors and Collaborators
Carol Davila University of Medicine and Pharmacy
Ministry of Education and Research, Romania
Investigators
Principal Investigator: | Mircea Cinteza, MD, PhD | University of Medicine and Pharmacy "Carol Davila" Bucharest |
Principal Investigator: | Dragos Vinereanu, MD, PhD | University of Medicine and Pharmacy "Carol Davila" Bucharest |
Study Chair: | Andrei D Margulescu, MD | University of Medicine and Pharmacy "Carol Davila" Bucharest |
No
publications provided
Responsible Party: | University of Medicine and Pharmacy "Carol Davila" Bucharest ( Professor Mircea Cinteza, MD, PhD ) |
Study ID Numbers: | IDEI_242_2007, ID_216/PNII_242_2007 |
Study First Received: | January 26, 2009 |
Last Updated: | June 29, 2009 |
ClinicalTrials.gov Identifier: | |
Health Authority: | Romania: National Authority for Scientific Research |
Keywords
provided by Carol Davila University of Medicine and
Pharmacy:
pulmonary
edema |
Additional
relevant MeSH terms:
Signs and
Symptoms | Vascular
Diseases |
ClinicalTrials.gov
processed this record on April 21,
2010
2. Research team
Project Director, Principal Investigator – Prof. Dr. Mircea Cinteza
Principal Investigator – Prof. Dr. Dragos Vinereanu
Main researcher, study chair – Dr. Andrei Dumitru Margulescu
Researchers – Dr. Roxana Cristina Sisu, Dr. Maria Florescu, Dr. Alexandru Dan Corlan.
3. Young researchers – degree of involvement
Main researcher, study chair – Dr. Andrei Dumitru Margulescu
- PhD thesis is represented by this research protocol.
- Study Chair
- wrote the research protocol, patient screening and recruitment, data analysis, wrote scientific research abstracts and articles
- author and co-author of all the scientific articles and abstracts published or submitted – that were sustained form this Project
- training program sustained from this Project at the University Hospital of Wales (July – Oct 2008)
- first author of a research article that has been published in the J Am Soc Echocardiogr.
Researcher – Dr. Roxana Cristina Sisu
-.PhD fellow at University of Medicine and Pharmacy Carol Davila Bucharest
-.Partially wrote the research protocol, patient screening and recruitment, data analysis, wrote scientific research abstracts and articles
-.Co-author of the majority of articles and scientific abstracts that were published or submitted from this Project
-.Training in transesophageal and intraoperative echocardiography at the Cardiovascular Surgical Unit in Novara, Italy – sustained by this Project (May – July 2008).
Researcher – Dr. Maria Florescu
-.PhD fellow at University of Medicine and Pharmacy Carol Davila Bucharest
-.Partially wrote the research protocol, patient screening and recruitment, data analysis, wrote scientific research abstracts and articles
-.Co-author of the majority of articles and scientific abstracts that were published or submitted from this Project
4. Projects’ objectives – current stage
Stages of the project
-. Oct 2007 – Local Ethical Committee approval for beginning of the study
-. May 2008 – May 2009 – patient screening and recruitment
83 screened patientsà 51 included
The largest study of its kind ever performed
-. Feb 2009 – included in ClinicalTrials.gov
-. Jan 2009 – partial results on the first 20 patients submitted at ESC 2009 – published in Eur Heart J in Sept 2009
-. May 2009 – partial results on the subanalysis on cardiac dyssynchrony on the first 30 patients submitted at Euroecho 2009 – published in Eur J Echocardiogr dec 2009
-.Jun-Sep 2009 – analysis of the full data base
-. Sep 2009 final results on the subanalysis for cardiac dyssynchrony submitted at the World Congress of ACrdiology, Beijing, 2010 – accepted as oral presentation. Abstract to be published in Circulation.
-.Jan 2010 – original research article accepted in JASE. See full list of articles from this Project.
-. Final article in the latest stages of preparation
5. Results

6. Full-text articles and abstracts sustained by this Project
Full text articles:
1. Margulescu AD, Thomas DE, Ingram T, Vintila VD, Egan M, Vinereanu D, Fraser AG. Can isovolumic acceleration be used in clinical practice to estimate ventricular contractile function? Reproducibility and regional variations of a new non-invasive index. Journal of the American Society of Echocardiography 2010; 23(4):423-31
2. Siliste C, Vinereanu D, Margulescu AD, Cinteza M. Right ventricular outflow tract implantation of an active fixation defibrillation lead through a persistent left superior vena cava. Europace 2008;10:1454-5.
3. Margulescu AD, Sisu RC, Cinteza M, Vinereanu D. Noncardiogenic acute pulmonary edema due to severe hypoglycemia - an old but ignored cause. Am J Emerg Med. 2008;26:839.e3-6.
Abstracts sustained by this Project:
1.Margulescu AD, Sisu RC, Florescu M, Cinteza M, Vinereanu D. Longitudinal, but not global systolic dysfunction is a mechanism of acute hypertensive pulmonary edema. ESC Congress of Cardiology, Barcelona 2009. (published in Eur Heart Journal 2009;30:301-585, Abstract Supplement). Online ISSN 1554-2815 - Print ISSN 1520-765X
2.Margulescu AD, Thomas DE, Ingram T, Vintila VD, Egan M, Vinereanu D, Fraser AG. Can isovolumic acceleration be used in clinical practice to estimate ventricular contractile function? Reproducibility and regional variations of a new non-invasive index. Euroecho 2009, Madrid (published in Eur J Echocardiography vol 10 Supplement 2, S63). ISSN 1525 - 2167 (print)
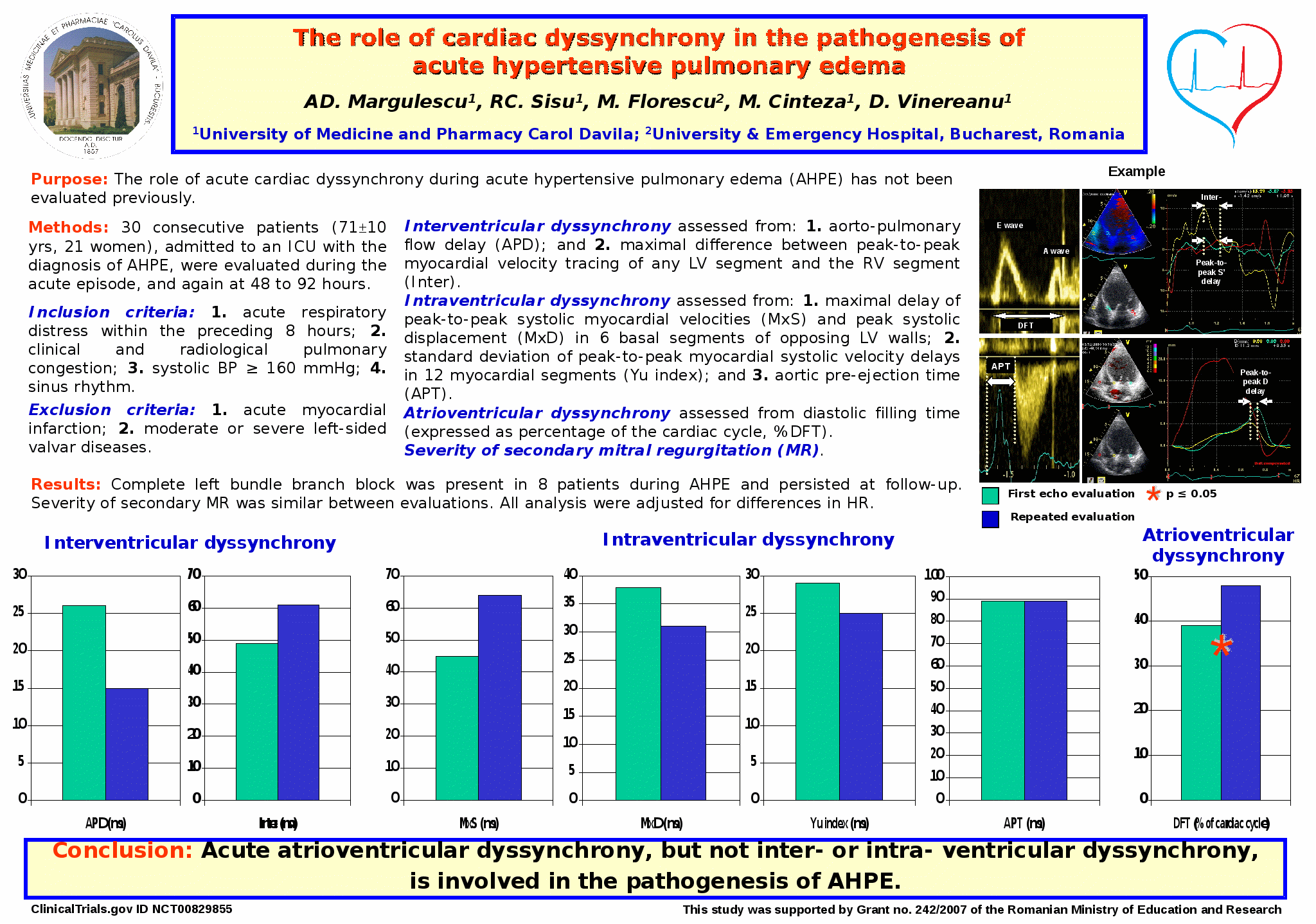
3.Margulescu AD, Sisu RC, Florescu M, Cinteza M, Vinereanu D. The role of cardiac dyssynchrony in the pathogenesis of acute hypertensive pulmonary edema – an echocardiographical study on 30 consecutive patients. Euroecho 2009, Madrid (published in Eur J Echocardiography vol 10 Supplement 2, S139). ISSN 1525- 2167 (print)
3.Margulescu AD, Sisu RC, Florescu M, Cinteza M, Vinereanu D. The role of cardiac dyssynchrony in the pathogenesis of acute hypertensive pulmonary edema. World Congress of Cardilogy - oral presentation, Beijing 2010. Abstract to be published in Circulation.